Abstract
Hematopoietic cells are arranged in a hierarchy where stem and progenitor cells differentiate into mature blood cells. Likewise, AML (Acute Myeloid Leukemia) is also hierarchical with leukemic stem and progenitor cells giving rise to more mature and differentiated blasts. Recent studies have shown that mitochondrial enzymes such as IDH2 can regulate AML stemness by altering metabolites that affect epigenetic marks. However, it is unknown whether mitochondrial metabolic enzymes can directly localize to the nucleus to regulate stemness in AML and normal hematopoietic cells. Here, we show that the mitochondrial enzyme, Hexokinase 2, localizes to the nucleus in AML and normal hematopoietic stem cells to maintain stemness.
We sought to identify mitochondrial metabolic enzymes that localize to the nucleus of stem cells, by evaluating the stem and bulk fractions from 8227 leukemia cells. 8227 leukemia cells are arranged in a hierarchy with functionally defined stem cells present in the CD34+CD38- fraction. We separated 8227 cells into CD34+CD38- and CD34-CD38+ populations by FACS sorting and prepared lysates of the nuclear and cytoplasmic fractions from each population. Using immunoblotting, we measured levels of mitochondrial enzymes in the subcellular fractions of each population. We discovered that the metabolic enzyme Hexokinase 2 (HK2) was increased in the nuclear fraction of 8227 stem cells compared to bulk cells. In contrast, other mitochondrial enzymes such as Aconitase 2 and Succinate Dehydrogenase B were not detected in the nuclear fractions. HK2 is an outer mitochondrial membrane protein that phosphorylates glucose to glucose-6-phosphate, thereby initiating glycolysis and the entry of glucose metabolites into the TCA cycle in the mitochondria. The nuclear localization of HK2 in mammalian cells has not been previous reported. We confirmed that 8227 cells have nuclear HK2 by confocal fluorescent microscopy and also demonstrated nuclear HK2 in AML cell lines (OCI-AML2, NB4, K562, and MV411) and primary AML samples.
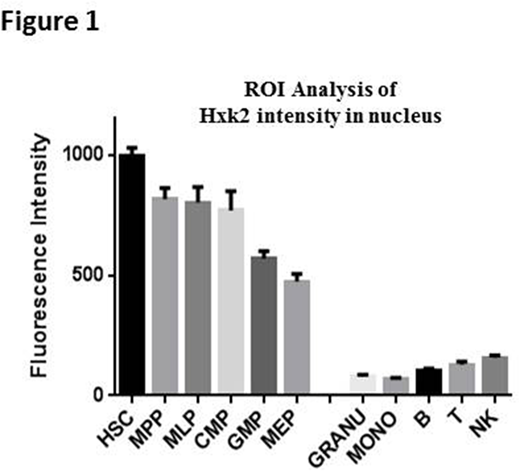
We also FACS sorted normal cord blood into populations of stem/progenitor (HSC, MPP, MLP, CMP, GMP and MEP) and differentiated (B cells, T cells, NK cells, Monocytes and Granulocytes) cells. The localization of HK2 in these cell fractions was measured by immunofluorescence and quantified by Metamorph and Imaris. Nuclear HK2 was detected in the stem/progenitor cells and progressively declined to minimal levels as the cells matured (Fig 1A).
The mitochondrial localization of HK2 is dependent on AKT-mediated phosphorylation of Thr-473 and inhibited by dephosphorylation by the phosphatase PHLPP1. We asked whether phosphorylation of HK2 regulates the nuclear abundance of HK2. Using AML2 cells, we showed that knockdown of PHLPP1 decreased the abundance of nuclear HK2, while inhibition of AKT increased HK2 in the nucleus.
Finally, we tested whether the nuclear localization of HK2 was functionally important to maintain stemness. We over-expressed HK2 tagged with nuclear localizing signals (PKKKRKV and PAAKRVKLD) in 8227 and NB4 leukemia cells. We confirmed the selective over-expression of HK2 in the nucleus of these cells by immunoblotting and immunofluorescence. Increasing nuclear HK2 did not alter the proliferation of the cells under basal conditions. However, increasing nuclear HK2 enhanced clonogenic growth and blocked retinoic acid-mediated cell differentiation.
In summary, we discovered that the unphosphorylated form of the metabolic enzyme HK2 localizes to the nucleus in malignant and normal hematopoietic stem cells and is functionally important to maintain stem/progenitor state. Thus, we define a new role for mitochondrial enzymes in the regulation of stemness and differentiation.
Schimmer:Medivir AB: Research Funding; Jazz Pharmaceuticals: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Otsuka Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal